PfizerBioNTech has initiated its application to the US Food and Drug Administration for full FDA approval of its Covid-19 vaccine for people ages 16 and older the companies said Friday. AstraZeneca Weighs Seeking Full US.
This shot will boost your immune response.

Fda booster shot approval. Aducanumab is the first Alzheimers drug approved by the FDA in almost 20 years. Liminal BioSciences Inc. Moderna said last month that its shot proved 100 percent effective in teens ages 12 to 17 enrolled in a late-stage clinical trial.
Full FDA approval would allow Pfizer to market its vaccine directly to consumers and could help make Pfizer Covid-19 booster shotssomething Pfizer CEO. Such as the Public Citizen have opposed approval. Plus the newest vaccine trial data how a lack of truck drivers could lead.
In addition the company is also planning a third booster shot. AZN COVID-19 vaccine works well as a third booster shot according to a study the Financial. In the US theres even an independent advisory committee which reviews data sets from both the FDA and drug companies before the agency makes its final decision on whether to greenlight a new shot.
Food Drug Administration FDA has approved Ryplazim plasminogen human-tvmh. AstraZeneca may skip asking the FDA for emergency-use authorization for its COVID-19 vaccine and instead pursue a full-fledged license to sell the shot. The company is the second vaccine maker after Pfizer and its partner BioNTech to seek full FDA approval.
The company is the second vaccine maker to seek full approval. Last week the company applied to the US FDA for full approval for its mRNA Covid-19 vaccine for use in people 18 and older. The plan is for every adult to get a booster shot eight months after you got your second shot he said.
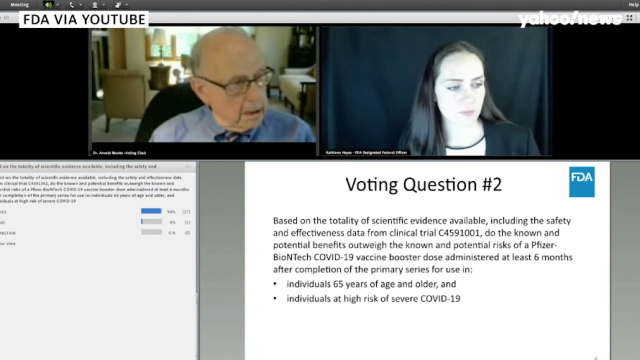
Emergency use authorization allows a vaccine to become available prior to full approval in the case of public health emergencies. The FDA will scrutinise the information to see if the vaccine meets stringent criteria for full licensure. In summary the FDA has not released specifics on size or timing for booster shot trials but with our sources we can verify clinical trial data is required and will study how well a booster shot.
The NYT reported on May 4 that the average number of people getting a first or single dose of a COVID vaccine each day had fallen by about 50 percent since April 13. Nearly 70 percent of Virginia adults have received at least one dose of a coronavirus vaccine less than three weeks from President Bidens goal to reach the milestone by July 4. LMNL Liminal BioSciences or the Company announced today that the US.
Shots are fully vaccinated without booster. What difference would full FDA approval make for COVID-19 vaccines. Currently no COVID-19 vaccine is fully approved by the FDA but three - Moderna Pfizer and the currently questionable Johnson Johnson - were given emergency use authorization by the agency.
May wait to apply for full approval by the FDA. Oxford University and AstraZeneca Plcs NASDAQ. MODERNA SEEKS FULL APPROVAL OF COVID SHOT The company is seeking full FDA approval of its vaccine for use in those 18 and older it announced Tuesday morning.
Moderna has asked the Food and Drug Administration for full approval of its coronavirus vaccine in people 18 and older. C OVID-19 vaccine maker Moderna has petitioned the Food and Drug Administration for full approval of its shots for use for adults 18 and older. Approval for Covid Shot Skipping Emergency-Use Application British drugmaker faces challenges gathering data.
Moderna has since expanded its research to test the vaccine in younger people. This along with full approval from the FDA is likely to help raise public confidence in the vaccinewhich is important in a time when vaccinations have slowed in the US. Moderna is the second Covid-19 vaccine maker to seek full approval following Pfizer and.
That would allow the company to.

Who Calls For Booster Moratorium To Get Poorer Countries Vaccinated

Oneida County Ready For Covid Booster Shots Once Fda Gives Permission

Fda Staff Say Pfizer Covid 19 Boosters May Not Be Needed But Do Improve Immunity Pharmalive
Update On Covid 19 Vaccine Booster Shots And Pfizer Biontech Comirnaty Covid 19 Vaccine Full Fda Approval 8 23 2021 Catalina Island Medical Center
Moderna Begins Submission Process For Fda Approval Of Its Covid Booster Shot Barron S